Costa Rica is the global leader in fresh pineapple exportation, accounting for 85 % of the U.S. pineapple market (Vargas Céspedes et al., 2018), and Costa Rica ranks first for the quantity of bananas produced per hectare (Vargas Céspedes et al., 2018). Such intensive agriculture is accompanied by a reliance on agrochemicals, especially pesticides. On a global scale, Costa Rica applies some of the highest quantities of pesticides per hectare of agricultural land (Vargas Castro, 2022), with banana and pineapple agribusinesses applying around 2.5 million recorded kgs of pesticides annually (Vargas Castro, 2022). Nearly all of the primary pesticides2 used for banana and pineapple agriculture are banned in at least 30 countries, including the European Union, because of their associated health risks (PAN, 2022).
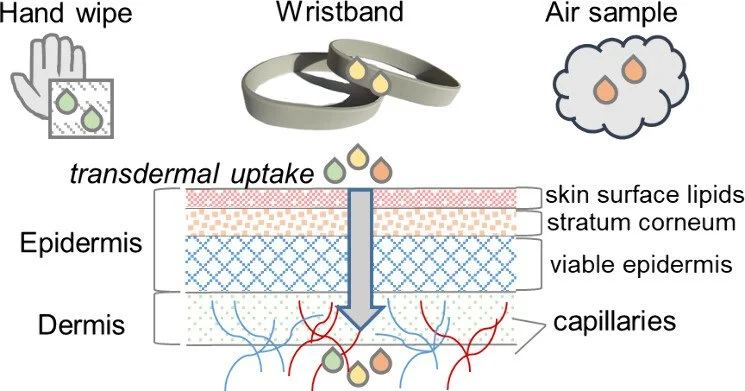
Assessment of personal exposure to semi-volatile organic compounds was facilitated using silicone wristbands (SWBs), an easy-to-use sampler that reflects total inhalation and dermal exposure from all the microenvironments and the activities in which the user was involved.
Uganda is known for its high levels of biodiversity and rich cultural heritage linked to this abundance of life. As a tropical Global South country, Uganda faces various environmental threats to biodiversity and human health, including climate change, deforestation, and habitat fragmentation. More recently, increasing chemical threats from pest control measures, vehicle emissions, and the gradual diffusion of household products have added the unintended biological side effects of industrial chemicals to this list (Wang et al., 2019). According to the Global Pesticide Use and Trade (GIoPUT), the use of pesticides in low and middle-income countries has increased substantially and has been underestimated (Shattuck et al., 2023). As a growing agriculture-based economy with a rapidly increasing human population, Ugandans are expected to increasingly face health risks from chemicals related to this development, including pesticides used in farming and flame retardants used in consumer goods.
Occupational exposure to semi-volatile organic compounds (SVOCs) among various population groups has garnered insufficient attention. We investigated the occupational exposures of waste disposal workers and daily exposure of university students to phthalates (PAEs), organophosphate esters (OPEs), and polycyclic aromatic hydrocarbons (PAHs) using polydimethylsiloxane (PDMS)-based passive sampling combined with machine learning-driven serum concentration predictions. The SVOC exposures of students varied depending on their professional activities, e.g., experiments, but dormitories emerge as a significant source.
Exposure to airborne contaminants has been linked to approximately nine million global deaths annually and to the onset of chronic diseases, including lung cancer and allergies (Lelieveld et al., 2020; Sonne et al., 2022). The contaminants include semi-volatile organic compounds (SVOCs), volatile organic compounds (VOCs) and particulate matter (Okeme et al., 2023).
Agricultural pesticide use has recently been linked to reduced bumble bee fitness across Europe (Nicholson et al. 2024) and declines in occupancy of hundreds of wild bee species in the United States (Guzman et al. 2024). For more than 50 years, there have been efforts to reduce the number of pesticide applications and to increase the use of more selective pesticides through integrated pest management (IPM) programs (Ehler 2006), yet the use of pesticides has increased worldwide (Bernhardt et al. 2017).
Introduction
Humans encounter an extensive array of natural and synthetic chemicals daily. Common exposure routes include dietary and nondietary ingestion, inhalation, and dermal absorption. Of these, dermal absorption has been historically neglected and underestimated due to challenges in assessing how exogenous chemicals penetrate the skin. (1,2) Additionally, fractional absorption (i.e., an assumption that a somewhat arbitrary fraction of chemical concentration will penetrate the skin) has been commonly and erroneously used for estimating dermal uptake. (3) To address transdermal permeation of semivolatile organic compounds (SVOCs), Weschler and Nazaroff presented two methods for calculating dermal uptake: air-to-skin transport and hand wipes as measures of skin-surface lipid concentrations. (1) These methods have been used to estimate dermal exposure for several SVOC classes including pesticides, polycyclic aromatic hydrocarbons, phthalates, and polychlorinated biphenyls (PCBs). (1,2,4−6) They are described as frameworks for determining the dermal contribution to exposure within an order of magnitude; this is due to an explicit understanding that these dermal estimates rely on the accuracy of thermodynamic parameters.
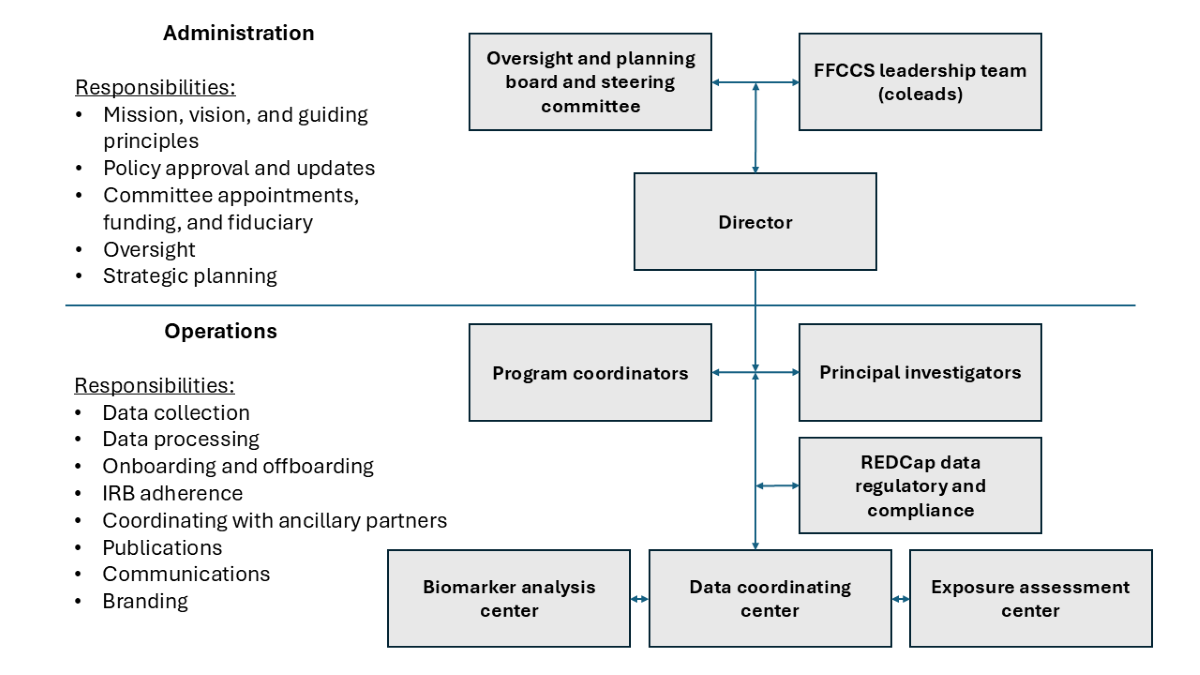
The Fire Fighter Cancer Cohort Study (FFCCS) is designed to address the distinctive challenges of conducting research in the fire service because it is a community-engaged partnership of academic and governmental research centers with the fire service. In community-engaged research, community representatives and researchers share all aspects of the research process, including co‐learning and reciprocal transfer of expertise, shared decision-making, and mutual ownership of the processes and products of the research [19]. Community-engaged research with firefighters is critical to not only answer their research questions but also to effectively share this information with the broader fire service community and inform actions to protect their health. Furthermore, the prospective nature of the FFCCS allows for the collection of survey data and biological samples before the development of disease. The goal of the FFCCS is to work with the fire service to advance firefighter cancer control and prevention, as well as evaluation and prevention of other health conditions.
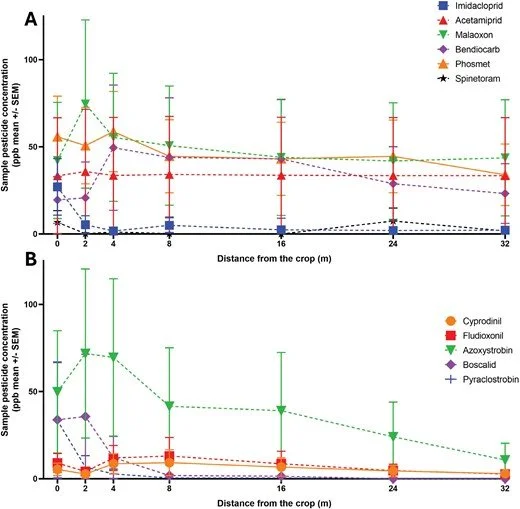
Exposure characterization and quantification of pesticides among workers and residents living close to agricultural fields remains of great interest, mostly due exposure to a large amount of different sprayed compounds, but also due to their potential health effects (Dereumeaux et al 2020). In the Netherlands, a recent study showed that homes close to flower bulb fields have increased pesticide concentrations in dust and air (Oerlemans et al 2021, Figueiredo et al 2022). These findings, however, are limited by the measurement period and don’t necessarily reflect long-term personal exposure. Characterization of exposure through human biomonitoring is also limited, mainly due to personal variability, rapid excretion, metabolism and analytical challenges to reliably quantify a large variety of pesticides. On the other hand, silicone wristbands provide a possible solution to measure personal pesticide exposures, with the applicability to measure large numbers of pesticides over longer periods of time (Dixon et al 2019, Doherty et al 2021). Wristbands are passive samplers with low-impact and low-costs, and can be used for long-term personal exposure assessments in the general population (O’Connell et al 2014). Due to their non-invasive and easy to implement nature, wristbands could also be applied in less-studied and vulnerable populations, such as children or farm workers in low income countries (Travis et al 2020, Fuhrimann et al 2022, Running et al 2023, Veludo et al 2024). While most studies have a maximum wearing time of 30 days (Samon et al 2022), for this study, the wristbands were worn for longer periods (average 60 days). Wristbands will continuously bind and sequester pesticides, providing a time-weighted average during the time of wearing (Dixon et al 2020). This allows for detection of less often applied pesticides and provides aggregated cumulative exposure estimates. This study, initially mention elsewhere (Ottenbros et al 2023), describes exposure to a wide range of pesticides measured by silicone wristbands, providing insight in the applicability of using wristbands for pesticide mixture exposure assessment.
This study of children’s everyday chemical contaminant exposure and its associations with race, income, and brain structure produced four primary findings. First, as predicted, children were exposed to all six classes of chemical contaminants under study. Of the frequently detected compounds, the chemical contaminants detected at the highest concentrations were phthalates and alternatives, closely followed by OPEs.
The increasing concern about environmental pollution has prompted research on human exposure to harmful organic contaminants of different characteristics. The results obtained from the analysis of environmental samples, e.g. water, air, sediments and biota, provide a comprehensive overview of the accumulation of these pollutants in outdoors (Kurt-Karakus et al., 2018; Zou et al., 2018; Wania and Shunthirasingham, 2020; Huang et al., 2022; Vallecillos et al., 2024). Indoor pollution has also been a key area of focus for the scientific community. Studies have focused on the determination of volatile organic compounds (VOCs) and semi-VOCs in dust or air samples from workplaces, schools and houses (Ninyà et al., 2022; Núñez et al., 2022).
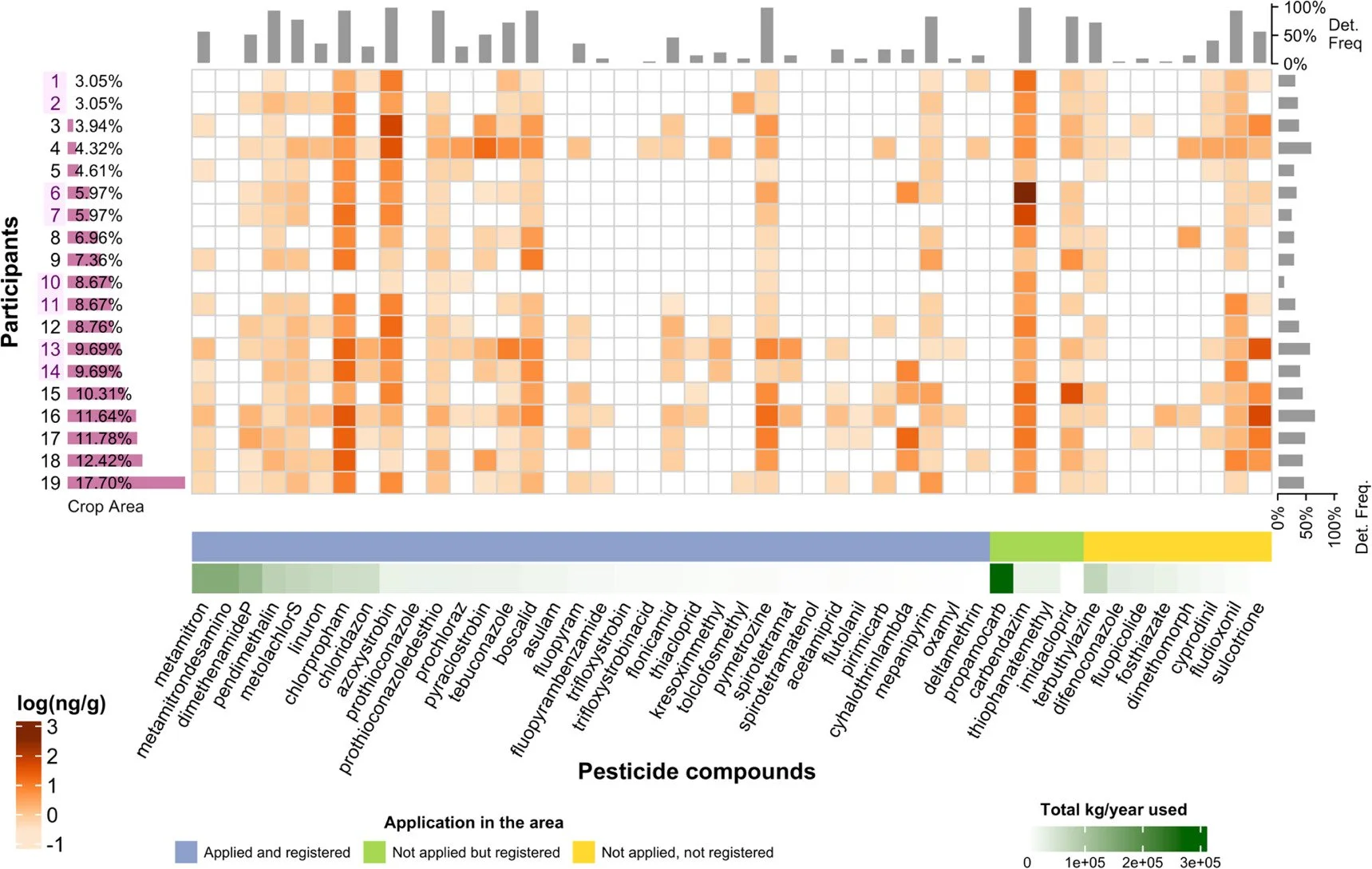
A crucial consideration in these studies is that most of them focus on collecting samples from a specific location. However, people move from place-to-place multiple times every day, and these continually changing locations make environmental samples from a single location unrepresentative. Starting in 2014, researchers began looking into the use of silicone wristbands (SWBs) for passive sample collection, which were presented as a comfortable, simple, and economical alternative (O'Connell et al., 2014; Anderson et al., 2017). In addition, as reported by Samon et al. (2022), SWBs not only take into account exposure to airborne pollutants but also other routes of exposure such as dermal contact. Semi-VOCs can also be transferred by particles adhering to skin or clothing or by the use of everyday products (personal care products, cleaning products, etc.). SWBs has been proved to effectively identify toxic compounds specific to tobacco, such as nicotine, and to monitor exposure to these compounds (Quintana et al., 2019). They have also been used as a monitoring method to determine semi-VOCs such as brominated flame retardants (BFRs), organophosphorus esters (OPEs), PAHs and phenols, for instance, in occupational environments and to evaluate exposure in children (Romanak et al., 2019; F. Wang et al., 2020; Hamzai et al., 2022; Levasseur et al., 2022). SWBs have also been used as sensitive passive samplers to detect population exposure to pesticides present in the diet and environment (Aerts et al., 2018; Donald et al., 2016). Increased research into the use of SWBs has led to comparisons between their use and that of other personal passive samplers, such as hand wipes and household dust, to determine the presence of various compounds (Hammel et al., 2020; Levasseur et al., 2021). Non-invasive human matrices (sweat, saliva and urine) are suitable for exposure to more polar compounds such as pesticides, illicit drugs and antibiotics, among other (Genuis et al., 2016; Brasier et al., 2020; Sotom et al., 2023). Unlike the previously mentioned passive samplers, which are cumulative over time, SWBs enable you to know the specific duration of your exposure to the pollutant.
The amount of waste electrical and electronic equipment (WEEE or e-waste) generated annually by the human population is the fastest growing waste stream from the planet (Forti et al., 2020, Liu et al., 2023). E-waste includes a wide range of materials from devices such as computers, household appliances, medical equipment and photovoltaic panels, as well as their components such as batteries, printed circuit boards, or cables (European Commission, 2022, Preet and Smith, 2024, Sarkar et al., 2024). The driver behind the ongoing rapid growth of the e-waste recycling industry is that many materials have an elevated value and can be reused as ceramics, plastics and metals. However, the chemically complex nature of some of the materials being recycled can involve the release of pollutants during processing. These pollutants can include heavy metals (Pb, Cd or Hg, among others) or persistent organic pollutants (POPs) such as polybrominated diphenyl ethers (PBDEs), or organophosphate esters (OPEs) (Okeke et al., 2024, Sindiku et al., 2015), thus potentially contaminating the environment around recycling facilities and exposing nearby populations to carcinogenic substances (Asante et al., 2019, Lu et al., 2023, Ma et al., 2022).
Firefighters work in conditions that are often associated with stress, dehydration, exhaustion, and excessive physical workload. They are also exposed to combustion emissions and heat. In addition, firefighters experience an elevated risk of injuries, chronic diseases, and certain types of cancer [1]. In a significant development, the International Agency for Research on Cancer (IARC) classified “occupational exposure as a firefighter” as a Group 1 carcinogen, i.e., a known human carcinogen [2]. This classification, supported by a growing body of evidence [1,3,4], underscores the importance of identifying the chemical contaminants firefighters are exposed to and understanding their correlations with increased incidence of certain cancers.
Fires, including municipal structural fires and wildfires, involve the combustion of a vast range of materials such as organic biomass (e.g., wood), electronics, construction materials, furnishings, textiles, and consumer products. Combustion of these materials produces and/or releases complex and hazardous emissions containing gases (e.g., carbon monoxide, nitrogen dioxide, and hydrogen cyanide), volatile organic compounds (VOCs) (e.g., benzene, styrene, and formaldehyde), dust and combustion-derived particulate matter (e.g., PM10), fibers (e.g., asbestos), metals (e.g., Sb, Pb, Cd, As), and semi-volatile organic compounds (SVOCs) (e.g., flame retardants, per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), dioxins, furans, and polycyclic aromatic hydrocarbons (PAHs)). For detailed information regarding the chemical composition of combustion emissions and the toxicological properties of substances in combustion emissions, the reader is referred to IARC Monograph 132 [2].
Flame retardants (FRs) are compounds added to consumer products and building materials to comply with flammability standards. One important group of FRs is the polybrominated diphenyl ethers (PBDEs), which are widely used in building materials and consumer products. Concerns relating to their environmental persistence, bioaccumulation, and toxicity resulted in the prohibition of marketing or use of Penta- and OctaBDE by the European Union in 2003 [5]. Penta and OctaBDE were later phased out in the USA in 2005, followed by Canada in 2008. DecaBDE was phased out in the USA in 2013 [6]. As alternatives to PBDEs, organophosphate ester flame retardants (OPFRs) are incorporated into electronic products, plastics, textiles, and furniture to reduce their flammability [6]. In addition, OPFRs are increasingly utilized as plasticizers or in personal care products such as nail polish and hair sprays. Exposure to OPFRs has been linked to genotoxic and endocrine system effects, developmental effects, and certain cancers [7,8].
Wristband personal samplers enable human exposure assessments for a diverse range of chemical contaminants and exposure settings with a previously unattainable scale and cost-effectiveness. Paired with nontargeted analyses, wristbands can provide important exposure monitoring data to expand our understanding of the environmental exposome. Here, a custom scripted suspect screening workflow was developed in the R programming language for feature selection and chemical annotations using gas chromatography–high-resolution mass spectrometry data acquired from the analysis of wristband samples collected from five different cohorts. The workflow includes blank subtraction, internal standard normalization, prediction of chemical uses in products, and feature annotation using multiple library search metrics and metadata from PubChem, among other functionalities. The workflow was developed and validated against 104 analytes identified by targeted analytical results in previously published reports of wristbands. A true positive rate of 62 and 48% in a quality control matrix and wristband samples, respectively, was observed for our optimum set of parameters. Feature analysis identified 458 features that were significantly higher on female-worn wristbands and only 21 features that were significantly higher on male-worn wristbands across all cohorts. Tentative identifications suggest that personal care products are a primary driver of the differences observed.
While the field of environmental health science has traditionally focused on characterizing environmental contaminants and limiting exposure to substances harmful to human health, there is increasing interest in quantifying the exposome and addressing individualized health concerns related to environmental exposures (Baccarelli et al., 2023). The exposome is characterized as an individual’s lifetime exposures to multiple types of stressors and the biological implications of these exposures (Wild, 2005, Wild, 2012). Improved exposure characterization is now needed to guide intervention efforts to alleviate specific stressors and exposure-related health outcomes. To that end, the term “precision environmental health” has been used to describe efforts combining four key areas of research to develop individual interventions to prevent disease (Baccarelli et al., 2023): 1) understanding disease mechanisms of environmental agents, 2) using ‘omics data to predict risk, 3) accounting for heterogeneity of susceptibility among individuals, and 4) developing biomarkers. One way to achieve increased precision in environmental health studies is to integrate advanced biomonitoring techniques and social determinants of health, allowing for more holistic determination of cumulative exposure and risk.
Exposure to per- and polyfluoroalkyl substances (PFASs) primarily occurs via consumption of contaminated drinking water and food; however, individuals can also be exposed dermally and via inhalation indoors. This study developed an analytical method for measuring volatile PFASs in silicone wristbands and used them to assess personal exposure in a Midwestern community (n = 87).
Accurate assessment of personal chemical exposures is critical to the advancement of environmental health sciences. Human biomonitoring of environmental chemicals has been traditionally considered as a gold standard, (Sexton et al., 2004) but many factors including costs, lab methods, and biospecimen storage and transport procedures can limit our ability to identify biomarkers that are sensitive, specific, and easy to measure (Mayeux, 2004). Biospecimen collection and storage under the proper conditions can be challenging and costly (Samon et al., 2022). Of particular concern for vulnerable populations including children, the collection of biospecimens may require invasive procedures such as an intravenous blood draw. Additionally, biomarkers for non-persistent chemicals with short half-lives may be unreliable without multiple samples and unreasonably short intervals between biospecimen collections (Aylward et al., 2014).
A novel approach to assess exposure to synthetic pyrethroids includes the use of silicone wristbands (WBs). In this pilot study completed on (n = 24) volunteers, comprising a week-long sampling period, paired urine samples (metabolites), and WBs (native compounds) were analyzed.
Ninety-two total chemicals were detected, with eight chemicals not previously reported in firefighter exposure studies. Based on the magnitude and frequency of increased exposure in on-duty dog tags, relative to paired off-duty dog tags, five PBDEs and sec-butylbenzene were identified as potential occupational exposures; sec-butylbenzene and PBDE 49 have not previously been reported in firefighter exposure studies to the authors' knowledge. Multivariate analyses for these six compounds indicated that firefighter rank, fire response rates, and years in the fire service were poor indicators of increased occupational exposure. The greatest on-duty exposures to PBDEs were found in the low-call volume department among operational firefighters. Dog tags from firefighters at the high-call volume department accounted for 75% of PCB detections; one particular fire response may have contributed to this. Additionally, there was measurable similarity in total chemical exposure profiles between paired on- and off-duty tags for some firefighters.
Due to differences in chemical properties and half-lives, best practices for exposure assessment may differ for legacy versus novel brominated flame retardants (BFRs). Our objective was to identify the environment matrix that best predicted biomarkers of children's BFR exposures. Paired samples were collected from children aged 3–6 years and their homes, including dust, a small piece of polyurethane foam from the furniture, and a handwipe and wristband from each child. Biological samples collected included serum, which was analyzed for 11 polybrominated diphenyl ethers (PBDEs), and urine, which was analyzed for tetrabromobenzoic acid (TBBA), a metabolite of 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB).